The density of copper is 8.96 g/cm3, a fundamental property that plays a pivotal role in its widespread industrial and engineering applications. This comprehensive analysis delves into the intricacies of copper’s density, exploring its implications and showcasing its versatility across various sectors.
Copper’s high density contributes to its exceptional electrical and thermal conductivity, making it an indispensable material for electrical wiring, heat exchangers, and other critical components. Understanding the factors influencing copper’s density is crucial for optimizing its performance and tailoring it to specific applications.
Definition of Density
Density is a physical property of matter that describes the compactness of its structure. It is defined as the mass of a substance per unit volume.
Density is a scalar quantity, meaning it has only magnitude and no direction. It is typically expressed in units of grams per cubic centimeter (g/cm³).
Calculating Density
Density can be calculated using the following formula:
Density = Mass / Volume
Where:
- Mass is the amount of matter in an object, typically measured in grams (g).
- Volume is the amount of space occupied by an object, typically measured in cubic centimeters (cm³).
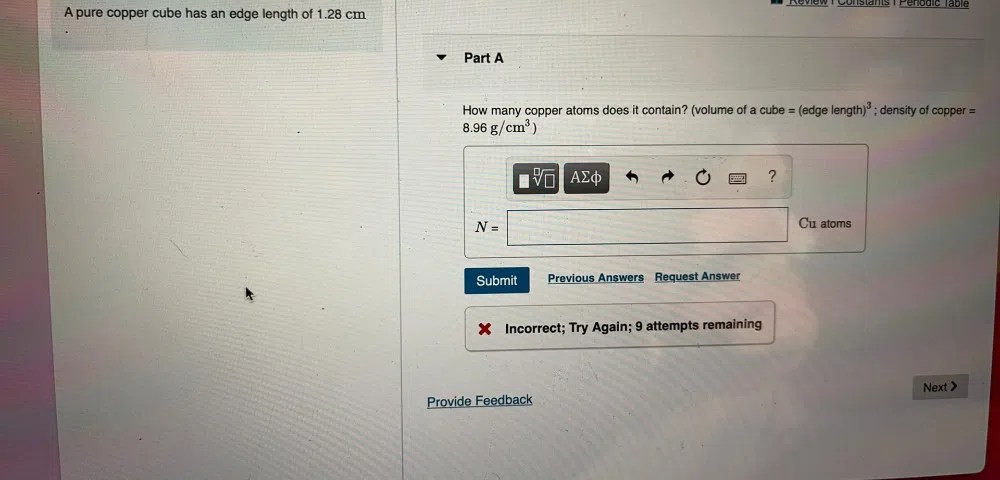
Density of Copper

Copper, an essential metal in various industries, possesses a remarkable density of 8.96 grams per cubic centimeter (g/cm3). This specific density value plays a significant role in determining the properties and applications of copper.
Significance of Copper’s Density
The density of copper contributes to its unique characteristics, including its strength, durability, and electrical conductivity. The high density of copper makes it a robust material, resistant to deformation and wear. Additionally, copper’s density enables it to conduct electricity efficiently, making it a valuable component in electrical systems and electronic devices.
Applications of Copper’s Density
The density of copper has practical implications in numerous applications. For instance, copper’s high density allows it to be used in construction as a structural material. Its durability and strength make it suitable for roofing, plumbing, and other building components.
Furthermore, copper’s density contributes to its use in electrical wiring, where its excellent conductivity ensures efficient transmission of electricity.
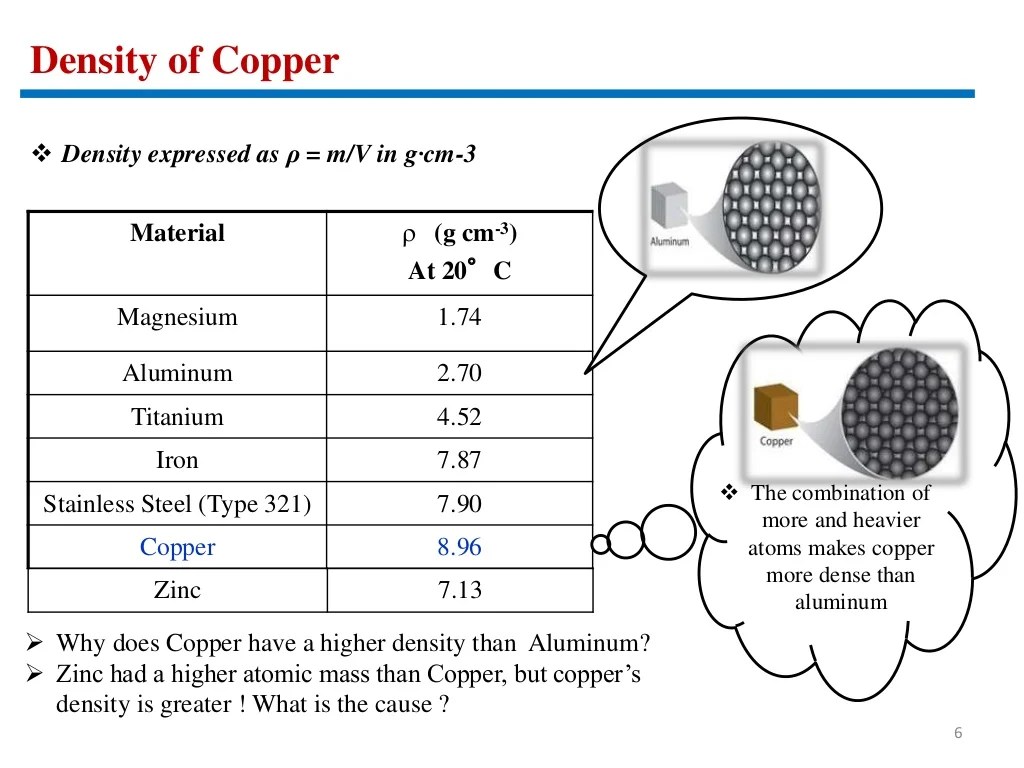
Comparison to Other Materials
The density of a material is an important property that affects its behavior in various engineering and industrial applications. Copper has a relatively high density compared to other common metals, which influences its suitability for specific purposes.
Density Comparison Table
The following table compares the density of copper to other common metals:
| Metal | Density (g/cm3) |
|---|---|
| Aluminum | 2.70 |
| Steel | 7.85 |
| Gold | 19.32 |
| Copper | 8.96 |
As can be seen from the table, copper is denser than aluminum but less dense than steel and gold.
Implications for Engineering and Industrial Applications
The differences in density among these metals have significant implications for their use in various applications. For example, the low density of aluminum makes it suitable for lightweight structures, such as aircraft and spacecraft, where weight reduction is crucial. Steel, on the other hand, is denser and stronger, making it ideal for structural applications where strength and durability are required.
Gold, with its high density and malleability, is often used in jewelry and other decorative applications.
Copper’s intermediate density makes it a versatile material with a wide range of applications. Its high electrical and thermal conductivity make it suitable for electrical wiring, heat exchangers, and other applications where efficient energy transfer is required. Its corrosion resistance and durability also make it a good choice for plumbing, roofing, and other applications where exposure to harsh conditions is a concern.
Factors Affecting Density
The density of copper is not a fixed value and can be influenced by various factors. Understanding these factors is crucial for controlling and manipulating the material properties of copper to achieve specific requirements.
Temperature, The density of copper is 8.96 g/cm3
Temperature has a significant impact on the density of copper. As the temperature increases, the atoms within the copper lattice gain energy and vibrate more vigorously, causing the material to expand. This expansion results in a decrease in density. The relationship between temperature and density is generally linear, with density decreasing as temperature increases.
Purity
The purity of copper also affects its density. Impurities, such as oxygen, sulfur, and other elements, can occupy interstitial sites within the copper lattice, disrupting the regular arrangement of atoms. This disruption can lead to a slight increase in the volume of the material, resulting in a decrease in density.
The extent of the decrease in density depends on the type and concentration of impurities present.
Applications of Copper Density
The density of copper is a significant factor in various industries and applications due to its unique properties. The high density of copper contributes to its exceptional electrical and thermal conductivity, as well as its resistance to corrosion and wear.
Electrical Applications
In the electrical industry, copper’s high density allows for the creation of compact and efficient conductors. The dense structure of copper enables it to carry high electrical currents with minimal resistance, making it ideal for use in power transmission lines, transformers, and electrical wiring.
Thermal Applications
Copper’s high thermal conductivity makes it an excellent material for heat exchange applications. In heat sinks, copper’s density allows for a compact design while effectively dissipating heat from electronic components. Additionally, copper is used in heat exchangers, boilers, and cookware due to its ability to transfer heat efficiently.
Automotive Industry
In the automotive industry, copper’s density contributes to the durability and performance of vehicles. Copper is used in electrical wiring, radiators, and brake systems, where its high density ensures optimal conductivity, heat dissipation, and resistance to wear and corrosion.
Density Measurement Techniques
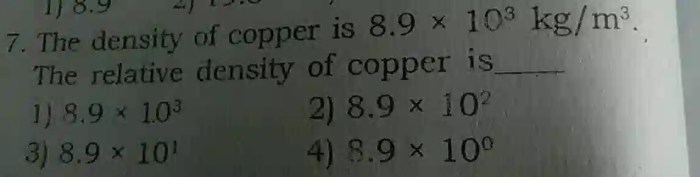
Measuring the density of copper is crucial for various applications, including material characterization, quality control, and scientific research. Several methods can be employed to determine the density of copper, each with its advantages and disadvantages.
Water Displacement Method
The water displacement method is a simple and widely used technique for measuring the density of solids. It involves immersing a known mass of copper in a graduated cylinder filled with water and measuring the volume of water displaced. The density is then calculated by dividing the mass of copper by the volume of water displaced.
Advantages:
- Simple and inexpensive
- Suitable for various shapes and sizes of copper samples
Disadvantages:
- Can be less accurate for small samples or samples with irregular shapes
- May not be suitable for porous materials or materials that react with water
Gas Pycnometry Method
Gas pycnometry is a precise method for measuring the density of solids and powders. It involves enclosing a known mass of copper in a calibrated chamber filled with a known gas, typically helium. The change in gas pressure is measured before and after the sample is introduced, and the density is calculated based on the Boyle’s law.
Advantages:
- High accuracy and precision
- Can be used for small samples and samples with irregular shapes
- Suitable for porous materials and materials that react with water
Disadvantages:
- More complex and expensive than the water displacement method
- Requires specialized equipment
Archimedes’ Principle Method
Archimedes’ principle method is another technique for measuring the density of solids. It involves suspending a known mass of copper in a liquid of known density and measuring the apparent weight of the sample. The density of the copper is then calculated using Archimedes’ principle, which states that the buoyant force acting on an object submerged in a fluid is equal to the weight of the fluid displaced by the object.
Advantages:
- Simple and inexpensive
- Can be used for various shapes and sizes of copper samples
Disadvantages:
- Less accurate than the water displacement method or gas pycnometry
- Requires a liquid that does not react with copper
Related Properties of Copper

In addition to its density, copper possesses several other physical properties that are closely related and influence its overall behavior.
Two notable properties are electrical conductivity and thermal conductivity, which are directly linked to the density of copper.
Electrical Conductivity
Copper is an excellent conductor of electricity due to its low electrical resistivity. This property is inversely proportional to density, meaning that the higher the density, the lower the electrical conductivity.
The high electrical conductivity of copper makes it an ideal material for electrical wiring, components, and other applications where efficient current flow is essential.
Thermal Conductivity
Copper is also a highly thermally conductive material, meaning it can transfer heat efficiently. This property is also inversely proportional to density, similar to electrical conductivity.
The high thermal conductivity of copper makes it suitable for heat sinks, cookware, and other applications where efficient heat transfer is required.
Query Resolution: The Density Of Copper Is 8.96 G/cm3
What is the significance of copper’s density?
Copper’s high density contributes to its exceptional electrical and thermal conductivity, making it an ideal material for electrical wiring, heat exchangers, and other critical components.
How can the density of copper be measured?
The density of copper can be measured using various techniques, including the Archimedes’ principle method, pycnometry, and X-ray diffraction.
What factors can affect the density of copper?
The density of copper can be influenced by factors such as temperature, purity, and alloying elements.