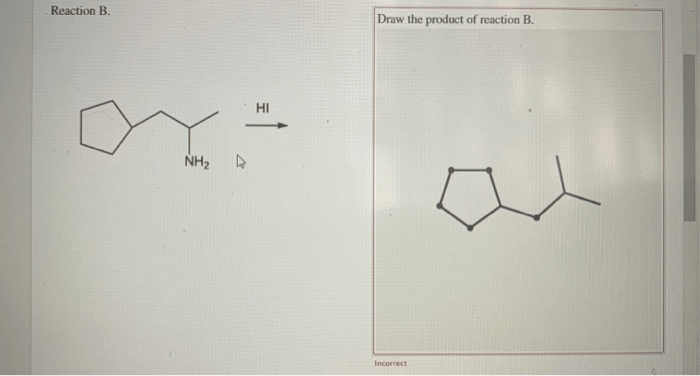
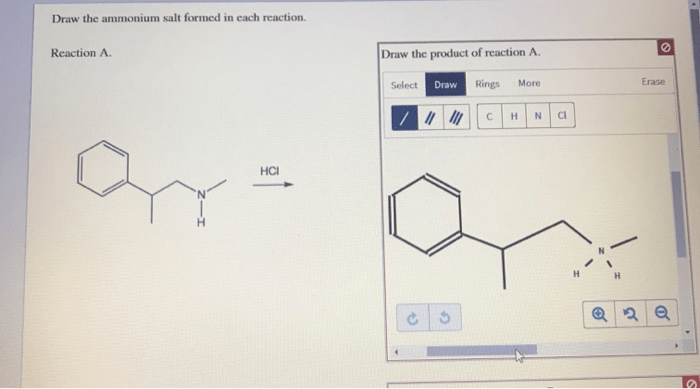
Draw the ammonium salt formed in each reaction. – Drawing the ammonium salt formed in each reaction is a crucial step in understanding the chemistry of these reactions. Ammonium salts are ionic compounds that contain the ammonium ion (NH4+). They are typically formed when an acid reacts with a base.
In this article, we will explore the chemical reactions involved in forming ammonium salts, their properties, and their applications.
Ammonium salts are white, crystalline solids that are soluble in water. They are typically odorless and have a slightly bitter taste. Ammonium salts are used in a variety of applications, including as fertilizers, food additives, and pharmaceuticals.
Ammonium Salt Formation
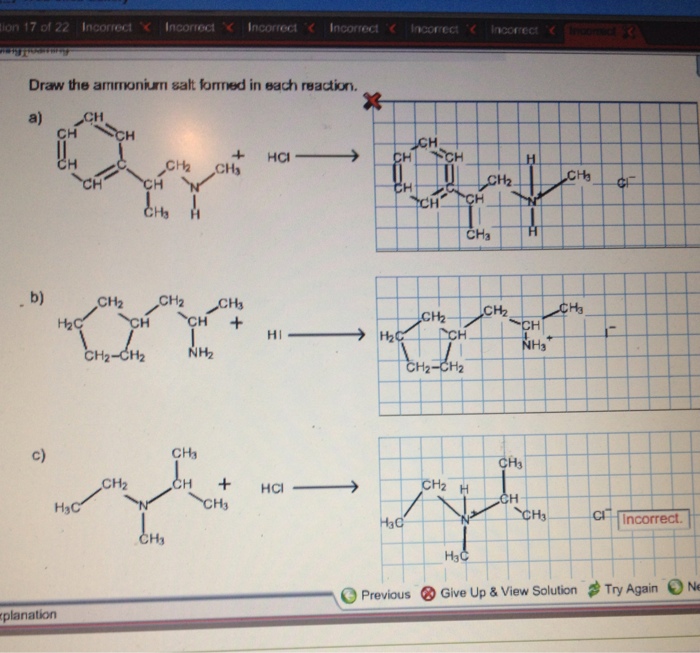
Ammonium salts are ionic compounds that contain the ammonium ion (NH 4+) as the cation. They are formed when a base, such as ammonia (NH 3), reacts with an acid, such as hydrochloric acid (HCl).
The chemical reaction involved in forming ammonium salts can be represented as follows:
NH3+ HCl → NH 4Cl
In this reaction, ammonia acts as the base and hydrochloric acid acts as the acid. The products of the reaction are ammonium chloride (NH 4Cl), an ammonium salt, and water (H 2O).
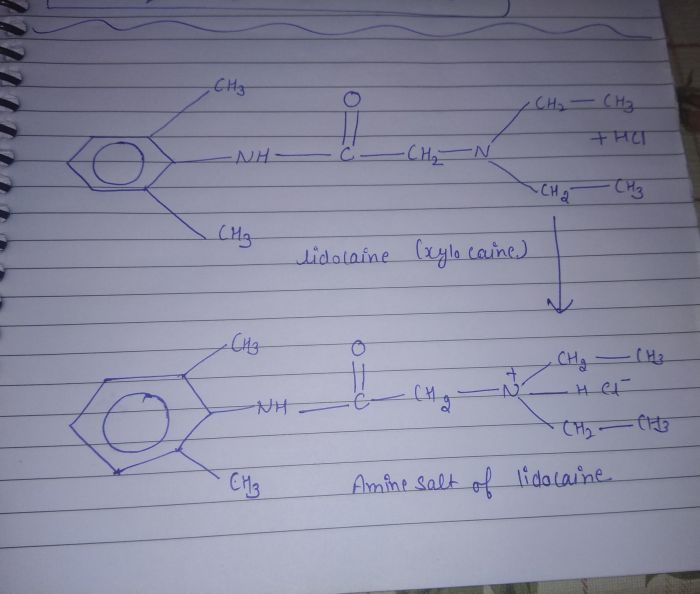
Ammonium salts can also be formed by the reaction of an amine with an acid. Amines are organic compounds that contain a nitrogen atom with a lone pair of electrons. When an amine reacts with an acid, the lone pair of electrons on the nitrogen atom is donated to the acid, forming an ammonium ion.
Examples of Reactions that Yield Ammonium Salts
- Ammonia + hydrochloric acid → ammonium chloride
- Methylamine + sulfuric acid → ammonium sulfate
- Trimethylamine + nitric acid → ammonium nitrate
The ammonium ion in ammonium salts plays an important role in the reactions of these compounds. The ammonium ion is a weak acid and can react with bases to form ammonia and water. This reaction is known as the hydrolysis of ammonium salts.
Properties of Ammonium Salts
Ammonium salts are typically white or colorless solids. They are soluble in water and have a slightly bitter taste. Ammonium salts are also hygroscopic, meaning that they absorb moisture from the air.
The solubility of ammonium salts in water is due to the formation of hydrogen bonds between the ammonium ion and water molecules. The acidity of ammonium salts is due to the hydrolysis of the ammonium ion in water.
Ammonium salts are used in a variety of applications, including:
- As fertilizers in agriculture
- As preservatives and flavor enhancers in the food industry
- As active ingredients and excipients in the pharmaceutical industry
Applications of Ammonium Salts
Ammonium salts are used in a variety of applications, including:
Agriculture
Ammonium salts are commonly used as fertilizers in agriculture. The ammonium ion is a source of nitrogen for plants, which is an essential nutrient for plant growth. Ammonium salts are also less likely to leach out of the soil than other nitrogen fertilizers, such as nitrates.
Food Industry, Draw the ammonium salt formed in each reaction.
Ammonium salts are used as preservatives and flavor enhancers in the food industry. Ammonium chloride is used to prevent the growth of bacteria in meat and fish products. Ammonium sulfate is used to enhance the flavor of processed foods, such as soups and sauces.
Pharmaceutical Industry
Ammonium salts are used as active ingredients and excipients in the pharmaceutical industry. Ammonium chloride is used as an expectorant in cough syrups. Ammonium sulfate is used as a laxative and diuretic.
Safety Considerations: Draw The Ammonium Salt Formed In Each Reaction.
Ammonium salts are generally safe to handle, but they can be irritating to the skin and eyes. Ammonium salts should be stored in a cool, dry place away from direct sunlight. Ammonium salts should also be disposed of properly in accordance with local regulations.
Ammonium salts can be toxic if ingested in large quantities. The toxicity of ammonium salts is due to the release of ammonia in the body. Ammonia is a toxic gas that can cause respiratory problems and death.
Ammonium salts can also have a negative impact on the environment. Ammonium salts can leach into groundwater and contaminate drinking water supplies. Ammonium salts can also contribute to air pollution by releasing ammonia into the atmosphere.
Questions Often Asked
What is an ammonium salt?
An ammonium salt is an ionic compound that contains the ammonium ion (NH4+).
How are ammonium salts formed?
Ammonium salts are typically formed when an acid reacts with a base.
What are the properties of ammonium salts?
Ammonium salts are white, crystalline solids that are soluble in water. They are typically odorless and have a slightly bitter taste.
What are the applications of ammonium salts?
Ammonium salts are used in a variety of applications, including as fertilizers, food additives, and pharmaceuticals.
