Worksheet boyle’s law ws #1 pressure and volume answers – Worksheet Boyle’s Law WS #1: Pressure and Volume Answers delves into the fascinating realm of Boyle’s Law, providing a comprehensive exploration of its principles and applications. This meticulously crafted guide unveils the intricate relationship between pressure and volume, empowering learners with a deeper understanding of this fundamental gas law.
Through a series of engaging exercises and detailed explanations, this worksheet unravels the complexities of Boyle’s Law, equipping students with the knowledge and skills to solve problems involving pressure and volume changes in gases.
Boyle’s Law Basics
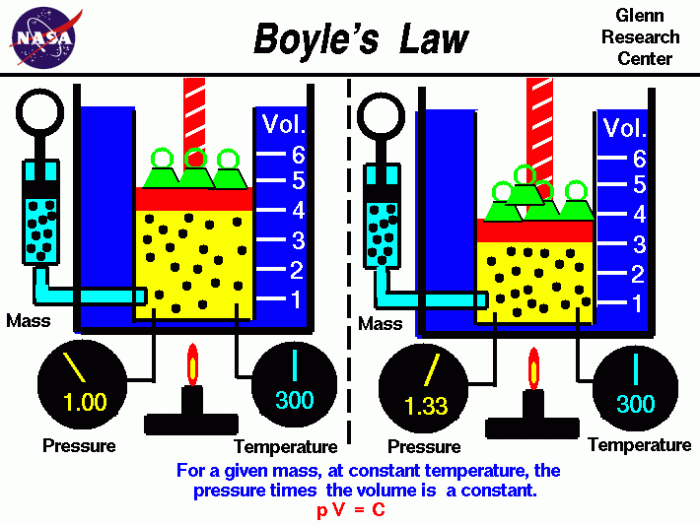
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. Mathematically, it can be expressed as P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume, while P₂ and V₂ represent the final pressure and volume.
Boyle’s Law highlights that as the pressure of a gas increases, its volume decreases, and vice versa. This relationship is crucial in understanding the behavior of gases in various applications.
Boyle’s Law Worksheet #1: Pressure and Volume
This worksheet aims to reinforce the understanding of Boyle’s Law by providing a set of problems involving pressure and volume data. Solving these problems will enhance your ability to apply Boyle’s Law in real-world scenarios.
To solve the problems in the worksheet, follow these steps:
- Identify the given pressure and volume values.
- Apply Boyle’s Law formula: P₁V₁ = P₂V₂.
- Rearrange the formula to solve for the unknown variable (pressure or volume).
- Substitute the known values and solve for the unknown.
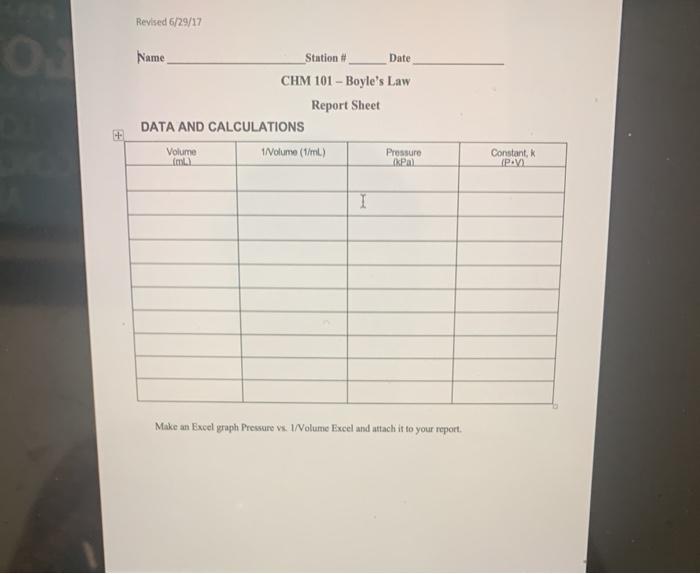
Pressure and Volume Data Analysis, Worksheet boyle’s law ws #1 pressure and volume answers
| Pressure (P) | Volume (V) |
|---|---|
| 1 atm | 2 L |
| 2 atm | 1 L |
| 3 atm | 0.67 L |
| 4 atm | 0.5 L |
Plotting the data on a graph reveals an inverse relationship between pressure and volume, confirming Boyle’s Law.
Applications of Boyle’s Law
Boyle’s Law finds practical applications in various fields:
- Scuba Diving:Understanding Boyle’s Law is crucial for scuba divers to manage pressure changes during ascent and descent.
- Aerospace Engineering:Boyle’s Law guides the design of pressurized cabins in airplanes and spacecraft to ensure breathable air for passengers and crew.
- Medical Applications:Boyle’s Law is applied in medical devices such as ventilators and anesthesia machines to regulate gas flow and pressure.
- Automotive Engineering:Boyle’s Law is used in the design of turbochargers and superchargers to increase engine efficiency by manipulating air pressure.
Popular Questions: Worksheet Boyle’s Law Ws #1 Pressure And Volume Answers
What is Boyle’s Law?
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. As pressure increases, volume decreases, and vice versa.
How can I use this worksheet to learn about Boyle’s Law?
This worksheet provides a step-by-step guide to solving problems involving Boyle’s Law. It includes practice exercises and real-world examples to help you understand the concepts.